EFSA’s Safety Opinion on Isoflavone-Containing Supplements for Post-Menopausal Women

17 Nov 2015
Conclusions give pause but no safety concerns
menopause is a natural-occurring life-stage women encounter, typically between the ages of 45 and 55, when estrogen levels decrease and menstruation ends. it can be accompanied by a variety of unpleasant symptoms, including hot-flashes, mood changes, and trouble sleeping, amongst others. although traditional hormone replacement therapy (hrt) has been a well-established protocol in western medicine for helping alleviate these symptoms, long-term use of hrt has been reported to be associated with certain adverse effects like increased risk of breast and endometrial cancer, as well as other side effects like nausea and other physical symptoms (shabir et al., 2013; burke et al., 2000). in light of these potential side effects, consumers have turned to alternative therapies, such as using isoflavone phystoestrogens. these are plant-based, endocrine-active substances that resemble human estrogen in chemical structure yet elicit a weaker response that can be consumed from the diet (soy-based, red clover, kudzu root) or through supplements containing extracts of these plants.
in light of the wide-spread availability of supplements containing these substances in the eu and the specific estrogenic properties of these products, the european food safety authority (efsa) was commissioned to conduct a systematic review of the available animal and human data associated with soy isoflavones/protein, daidzein rich isoflavones, genistein, and red clover to evaluate whether there are any similar safety concerns, with a specific focus on mammary, uterus, and thyroid organ effects.
based on the results of their review, efsa concluded that consumption of isoflavone-containing supplements was not associated with an increased risk of breast cancer, mammographic density, endometrial thickness or histopathological changes, or thyroid function. the only effect efsa noted was endometrial hyperplasia in 6 of 154 women consuming 150 mg of soy isoflavones per day for 5 years. however, efsa did not consider this to be an adverse effect.
due to wide variability in organ functions, receptor density and effects of receptor activation and differences between individual isoflavone effects and activity, efsa did not consider it appropriate to read-across results between different organ sites or extrapolate results from one isoflavone to another. that is why efsa was unable to derive single health-based guidance value or a safe intake level for food supplements containing isoflavones as a group. however, in comparing the highest doses evaluated without adverse effects in the 3 key organs and the longest duration of intake for the individual isoflavones, efsa did suggest dose and durations that could be considered as guidelines (see efsa's summary table below).

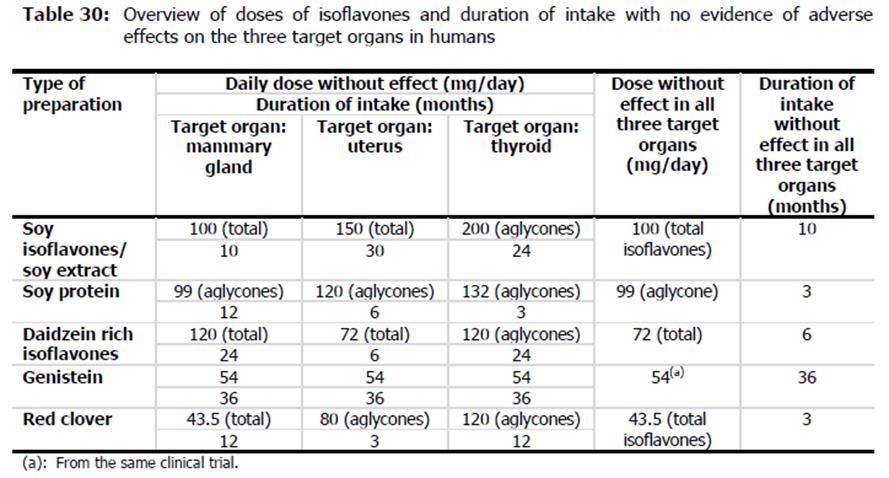
in comparison to products currently on the market in the eu, efsa found that most products recommended and provide doses comparable to those the agency considered safe. however, one issue efsa raised was that the products typically did not provide any instructions for the duration of use of these products. efsa also recommended more data would be beneficial on dosages (including harmonizing isoflavone content reporting in supplements) and durations.
so overall, although isoflavone supplements were generally considered safe and without any adverse effects, the precautionary principle is in effect when considering the safety of mammary, uterus, and thyroid organs based on the available data from which to derive safe use levels.
for full details on efsa's review and conclusions check out the opinion here: http://www.efsa.europa.eu/en/efsajournal/pub/4246
intertek scientific & regulatory consultancy provides regulatory solutions for food and supplement companies around the world (https://www.intertek.com/food/consulting/).
for any comments or questions related to food or supplement regulatory or safety issues please contact: food.sci-reg@intertek.com
references:
shabir et al., 2013: http://www.netjournals.org/pdf/irjmms/2013/3/13-029.pdf
burke et al., 2000: https://academic.oup.com/jn/article/130/3/664s/4686188
Blogger:
Tom Jonaitis,
Scientific and Regulatory Associate, Intertek