Essential Performance in Medical Devices: A Critical Component of Patient Safety
05 Nov 2024
Understanding the Role of Essential Performance in Ensuring Effective and Safe Medical Devices
Ensuring that a medical device performs as intended isn’t merely a goal or a “desired outcome.” It is a regulatory requirement. The concept of "Essential Performance" is central to this mandate, representing the critical characteristics of a medical device that must be maintained to ensure patient safety and effective operation. As the medical device industry evolves with increasingly complex technologies, understanding and defining Essential Performance has become more crucial than ever.
What Is Essential Performance?
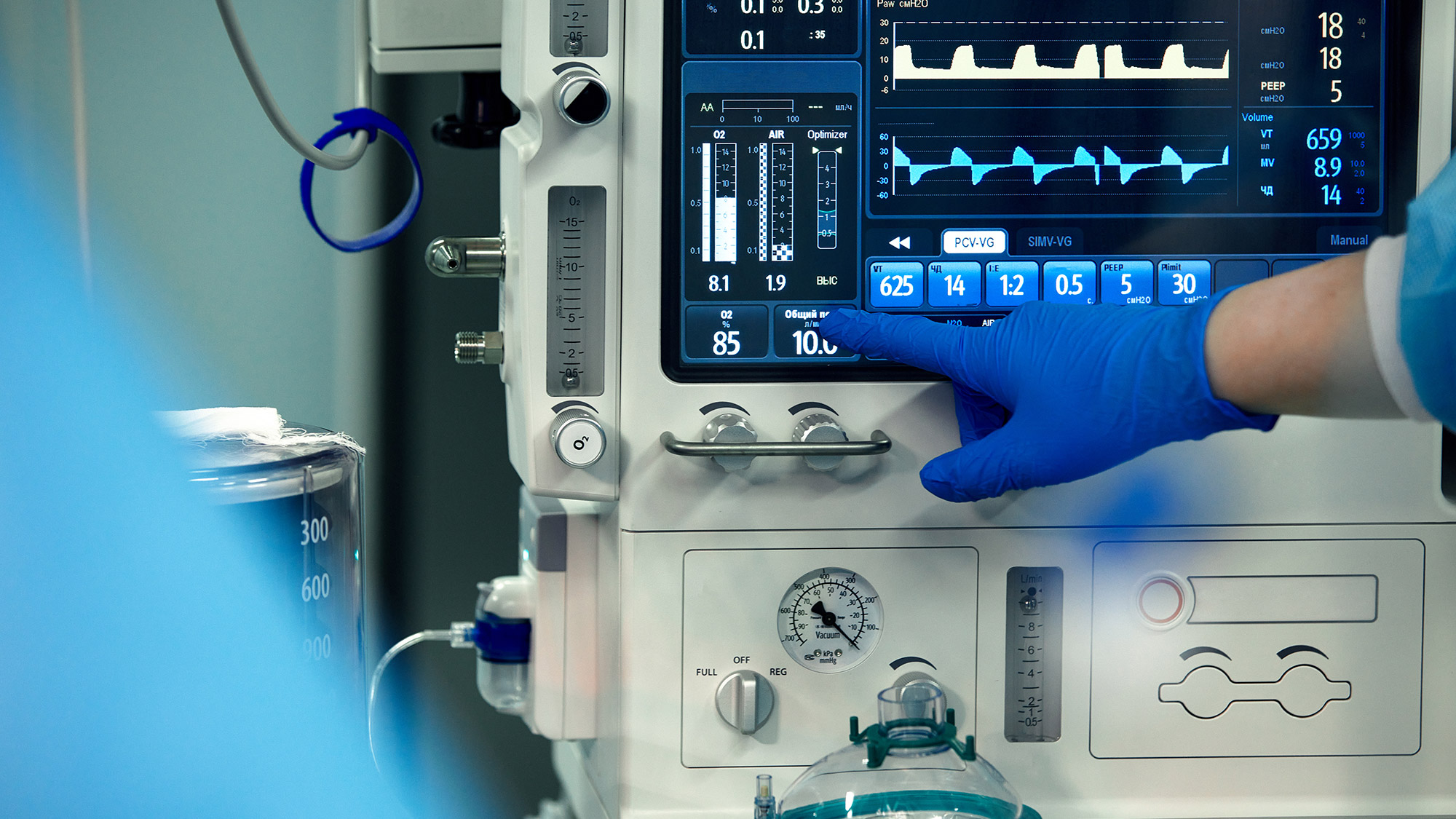
Essential Performance refers to the specific functions or characteristics of a medical device that are necessary for its safe and effective use. These are the features that must consistently perform within specified parameters to prevent harm to the patient or to ensure the device fulfills its intended medical purpose. The importance of Essential Performance cannot be overstated, as any failure in these critical functions could lead to severe consequences, including misdiagnosis, ineffective treatment, or even patient injury.
For instance, consider a blood pressure monitor. Its Essential Performance is its ability to accurately measure and display blood pressure levels. If the device fails to do so reliably, it could lead to incorrect diagnoses or improper management of a patient's health condition. Similarly, a surgical laser's Essential Performance lies in its ability to deliver precise and controlled energy to tissues, which is vital for the success of surgical procedures.
Essential Performance vs. Basic Safety: Understanding the Difference
While Essential Performance and Basic Safety are closely related concepts, they serve distinct purposes within medical device regulation. Basic Safety refers to the fundamental safety aspects of a device, such as protection against electrical shock, mechanical hazards, or radiation exposure. These are the minimum safety requirements that every medical device must meet to ensure it does not pose an immediate risk to patients or users.
On the other hand, Essential Performance goes beyond these basic safety measures. It focuses specifically on the performance characteristics that are critical to the device’s intended use. For example, while Basic Safety might address the electrical safety of a defibrillator, Essential Performance would focus on the device's ability to deliver the correct amount of energy needed to restart a patient's heart.
The distinction is crucial because a device can be safe in terms of basic operational hazards, but if it fails to perform its essential functions correctly, it can still pose a significant risk to patients.
Determining Essential Performance Using a Risk-Based Approach
The determination of Essential Performance is inherently tied to the intended use of the medical device. This process typically involves a risk-based assessment where manufacturers identify the potential risks associated with the device’s failure to perform certain functions. This assessment guides the identification of which performance characteristics are considered essential.
For example, a pacemaker’s Essential Performance includes maintaining a stable heart rate. If the pacemaker fails in this regard, it could lead to life-threatening conditions. Similarly, an insulin pump's Essential Performance is its ability to deliver precise doses of insulin as needed. Failure in this aspect could result in either hyperglycemia or hypoglycemia, both of which are dangerous for the patient.
Manufacturers must define Essential Performance early in the device design process, ensuring that these critical functions are thoroughly tested and validated before the device is brought to market.
The Role of Standards in Defining Essential Performance
Several standards, particularly the IEC 60601 series, provide guidelines for determining and testing Essential Performance. These standards require manufacturers to identify Essential Performance characteristics during the risk management process and ensure that these functions are maintained throughout the device’s lifecycle.
For instance, the IEC 60601-1 standard outlines general requirements for the safety and essential performance of medical electrical equipment. It mandates that manufacturers establish and document Essential Performance criteria and demonstrate that the device consistently meets these criteria under normal and fault conditions.
Some Part 2 standards, which are specific to certain types of medical devices, may also have additional requirements related to Essential Performance. These standards help guide manufacturers in defining what constitutes Essential Performance for their specific device and ensure that these critical functions are rigorously tested.
The Consequences of Failing to Meet Essential Performance
Failure to meet Essential Performance criteria can have severe consequences, both for patient safety and for the manufacturer. If a device does not perform its essential functions reliably, it could result in inaccurate diagnoses, incorrect treatments, or even device-related injuries.
For example, if a blood glucose monitor provides incorrect readings due to a failure in Essential Performance, a diabetic patient might administer the wrong dosage of insulin, leading to a potentially dangerous situation. Similarly, if a ventilator fails to maintain the necessary airflow due to an Essential Performance issue, the patient’s life could be at risk.
In addition to patient safety concerns, failing to meet Essential Performance can also lead to regulatory consequences. Devices that do not meet their Essential Performance criteria may be subject to recalls, regulatory actions, or even market withdrawal, leading to significant financial and reputational damage for the manufacturer.
Ensuring Compliance with Essential Performance Requirements
To ensure compliance with Essential Performance requirements, manufacturers must adopt a systematic approach throughout the device’s lifecycle. This includes:
- Identifying Essential Performance Early: Manufacturers should define Essential Performance characteristics during the initial design and development phase, based on the intended use and potential risks.
- Thorough Testing: Essential Performance must be tested under both normal and fault conditions to ensure that the device will perform reliably in all expected scenarios. This testing should be conducted according to relevant standards, such as IEC 60601.
- Ongoing Monitoring: Even after a device is launched, manufacturers must continue to monitor its performance in the field to ensure that it continues to meet its Essential Performance criteria. This may involve post-market surveillance, regular maintenance, and updates to the device as needed.
- Documentation and Reporting: Manufacturers must maintain detailed documentation of all Essential Performance testing and risk management activities. This documentation is crucial for regulatory submissions and for demonstrating compliance with Essential Performance requirements.
Summary: The Critical Importance of Essential Performance
Essential Performance remains a cornerstone of patient safety and device effectiveness. By understanding and defining the Essential Performance of their devices, manufacturers can ensure that their products not only meet regulatory requirements but also provide the highest level of care to patients.
The focus on Essential Performance underscores the importance of rigorous testing, risk management, and ongoing compliance throughout a device’s lifecycle. For manufacturers, this means that attention to Essential Performance is more than simply a regulatory obligation—it is a fundamental aspect of delivering safe, reliable, and effective medical devices that healthcare providers and patients can trust.
