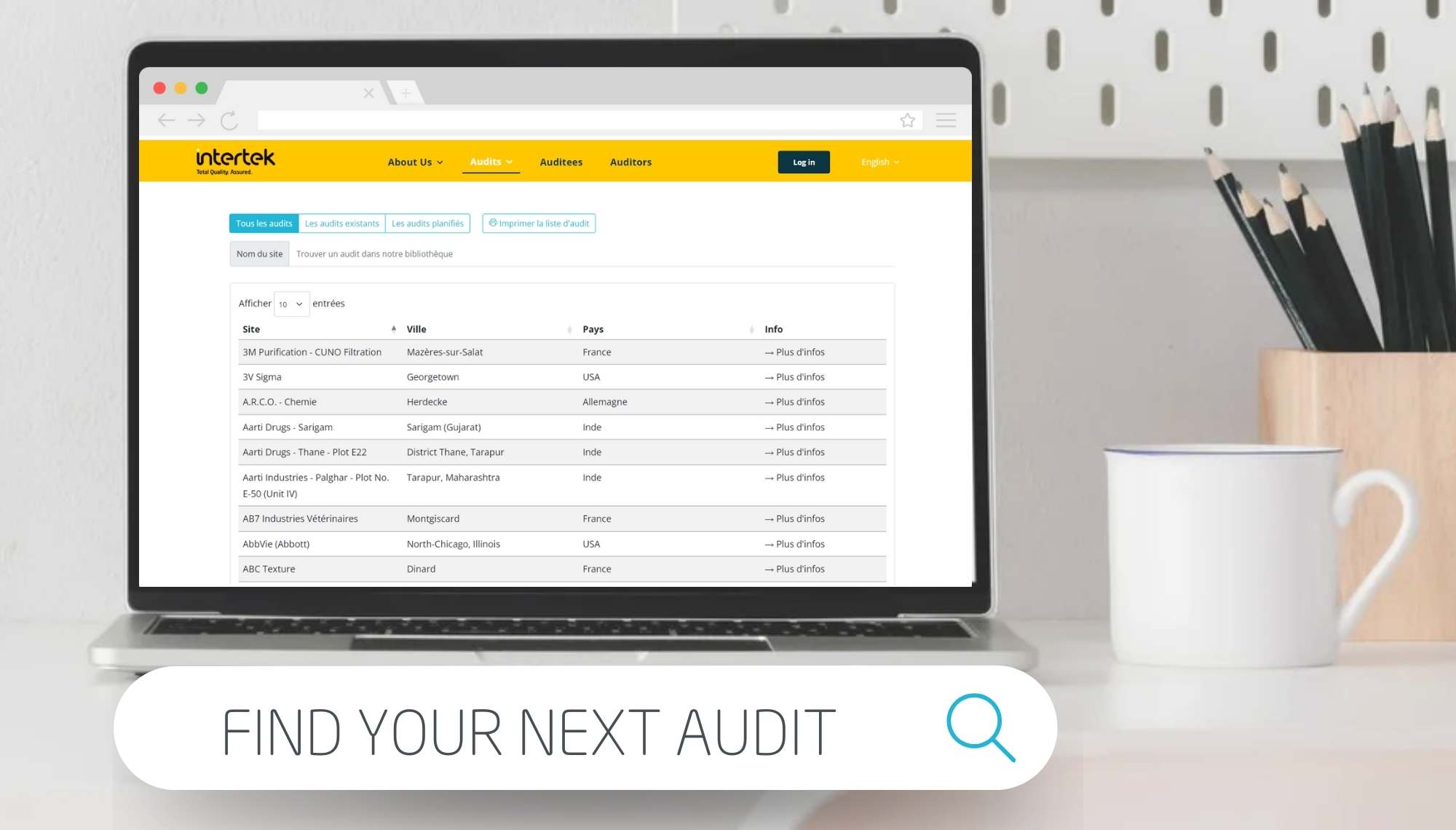
GMP auditing and inspection for the pharmaceutical supply chain. Flexible GXP pharmaceutical auditing solutions, helping you to improve control over quality for your complex supply chains. Our GMP auditors evaluate and monitor your suppliers, subcontractors, and service providers supporting your pharmaceutical product lifecycle
GMP audit and inspections for pharmaceutical industry suppliers and subcontractors remain a critical part of the drug development and manufacturing process.
An experienced GMP auditor who can evaluate and monitor supply chain stakeholders, can drive greater insight which enables you to improve control over quality. All regulatory agencies who set standards for the pharmaceutical industry have an expectation of Good Manufacturing Practice compliance, for example, across production supply chains. As a consequence, you will have an obligation to engage with your increasingly complex supply chain, and all supply chain actors including a multitude of suppliers, service providers and subcontractors.
Our Pharmaceutical Audit Experience
Our team of senior GMP auditors, who are located around the world, have in depth experience of conducting pharma audits to GMP, knowledge of the pharmaceutical regulatory standards, expectations and procedures. We conduct regulatory GxP audits in the pharmaceutical industry against the applicable regulatory texts or standards such as Good Manufacturing Practice (GMP), Good Distribution Practice (GDP), Good Laboratory Practice (GLP), Good Clinical Practice, (GCP), Good pharmacovigilance Practice (GVP).
We also audit against IPEC guidelines for Pharmaceutical excipients, ISO 22716 (Cosmetics — Good Manufacturing Practices), EFfCI GMP for cosmetic ingredients, ISO 15378 standard for packaging materials, ISO 9001 standard or EHPM Quality guide for food supplements. With robust internal procedures, our quality system and our auditor qualification process, driven by our Quality manager, is regularly audited by our clients with positive outcomes of reliability and robustness.
Flexible Audit Solutions
Intertek's flexible private or individual pharmaceutical audit solutions, shared audits, CAPA evaluation and follow-up, audit report purchase, remote or virtual audits and support for your internal audit program. We audit suppliers and manufacturers of APIs, excipients, packaging materials and other materials, subcontractors of manufacturing, packaging, analytical testing, service providers of clinical trials (CROs), pharmacovigilance, transporters, IT service, cleaning service and all other services.
How can we support your audit requirements?
The pharmaceutical industry continually faces increased focus and inspections by health authorities, coupled with travel restrictions and complex globalised supply networks. This all increases the demand on your supplier’s time to take part in GMP compliance audits and inspections and the need to meet ever-expanding regulatory expectations. With Intertek as your audit partner, we help you to overcome these challenges. Our solutions, such as our GMP audit services or our shared audit platform, can reduce demands on time and resources for all stakeholders in the supply chain by combining the requests to audit a particular supplier site. This in turn can help to optimise your budget through reduced audit costs.
Total Quality Assurance
We are ISO 9001 certified. Quality is at the heart of our organisation and we continuously focus on improving the performance of our services in order exceed expectations of our global clients. Our Total Quality Assurance expertise for pharmaceutical supplier and subcontractor auditing services is built upon over 15 years’ experience delivering audits with consistent quality and focus on detail over 5 continents. Our auditors have won the continuing trust of more than 1600 clients and can help you to identify and mitigate the intrinsic risk in your operations, supply chains and processes.
Pharmaceutical Solutions
A GMP audit is a comprehensive, third-party inspection of pharmaceutical production company or supplier in the pharmaceutical value chain. A GMP audit checklist aids the systematic audit of a drug manufacturing facility (either ingredients or finished products) and the GMP compliance audit process is aimed at identifying operational flaws and issues that impact quality.
What is GxP?
GxP refers to the Global quality guidelines, practices and regulations to ensure safe pharmaceutical and biopharmaceutical drug products and that they meet quality specifications and that processes and procedures during research and development, manufacturing, control, storage and distribution comply with specific standards. Industries such as the pharmaceutical, food, nutrition, cosmetics and medical devices are impacted by GxP.
Subscribe now
Subscribe to the Intertek Shared Audit newsletter